Electrochemical deposition of amorphous cobalt oxides for oxygen evolution catalysis†
Abstract
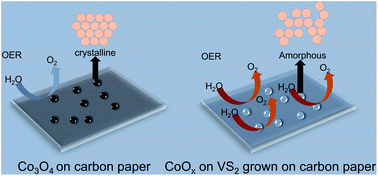
The oxygen evolution reaction (OER) is crucial in water splitting for hydrogen production. However, its high over-potential and sluggish kinetics cause an additional energy loss and hinder its practical application. The cobalt spinel oxide Co3O4 exhibits a high catalytic activity for the OER in alkaline solutions. However, the activity requires further enhancement to meet the industrial demand for hydrogen production. This paper presents an electrochemical deposition method to obtain cobalt oxides with a controllable crystallinity on carbon paper (CP). Usually, cobalt oxides grown on CP have a Co3O4 spinel oxide structure. The self-supported Co3O4/CP exhibited a considerable catalytic activity for the OER. When a VS2 layer grown on the CP beforehand by a hydrothermal method was used as substrate, the deposited cobalt oxides were in an amorphous state, denoted as CoOx/VS2/CP, which exhibited a higher OER activity and better stability than those of Co3O4/CP. The enhancement in the catalytic activity was attributed to the mixture formation of different types of cobalt species, including Co3O4, CoO, Co(OH)2, and metallic Co, because of the reduction by VS2. We also clarify the significance of the crystallinity of cobalt oxides in the improvement in the OER activity. This process can also be applied to the direct formation of other types of self-supported oxide electrodes for OER catalysis.


 Please wait while we load your content...
Please wait while we load your content...