Synthesis and crystal structure of a sulphur-bridged molecular hoop consisting of 5,7,12,14-tetrathiapentacene†
Abstract
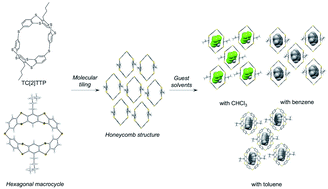
A cyclic dimer consisting of 5,7,12,14-tetrathiapentacene (TC[2]TTP) forms a new extended series of thiacalix[n]arenes, and was successfully synthesized by an intramolecular Friedel–Crafts-type condensation of the macrocyclic precursor. TC[2]TTP was characterized using 1H and 13C nuclear magnetic resonance and high-resolution mass spectrometry. Its hoop-shaped molecular structure was determined by X-ray crystallography. The two-tub-shaped TTP formed a hexagonal geometry via a sulphur linker, and TC[2]TTP adopted a honeycomb structure with columnar stacking in the crystal structure. Furthermore, TC[2]TTP exhibited crystal polymorphism, which incorporated appropriate organic solvents such as CHCl3, benzene, and toluene into its internal cavity. This suggests that TC[2]TTP is a candidate for the components of cavity-assembled porous solids based on molecular tiling.


 Please wait while we load your content...
Please wait while we load your content...