Selective preconcentration separation of Hg(ii) and Cd(ii) from water, fish muscles, and cucumber samples using recycled aluminum adsorbents†
Abstract
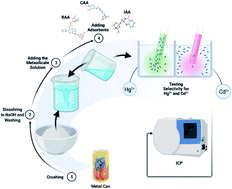
Modified aluminum scrap waste was used in the selective extraction of Hg(II), and Cd(II) ions. The aluminum scraps were modified with dibenzoylmethane, or isatoic anhydride, or 5-(2-chloroacetamide)-2-hydroxybenzoic acid. The modified aluminum sorbents were characterized by FT-IR, SEM, XRD, XPS, TGA, and elemental analysis. Modes of chelation between adsorbents and target metal ions were deduced via DFT. The highest adsorption capacity was observed for benzo-amino aluminum (BAA) toward Hg(II), which reached 234.56 mg g−1, while other modified sorbents ranged from 135.28 mg g−1 to 229.3 mg g−1. Under the optimized conditions, the BAA adsorbent showed a lower limit of detection (1.1 mg L−1) and limit of quantification (3.66 mg L−1) for mercury ions than other sorbents. The prepared aluminum adsorbents also exhibited significant selectivities for Hg(II) and Cd(II) ions in the presence of competing metal ions.


 Please wait while we load your content...
Please wait while we load your content...