Chemical redox-induced chiroptical switching of supramolecular assemblies of viologens†
Abstract
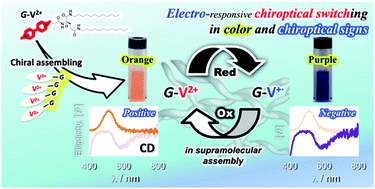
A chiral supramolecular assembly exhibiting redox-induced changes in its chiroptical properties was prepared using viologen-modified glutamide (G-V2+) derivatives. Achiral viologen moieties in the G-V2+ assembly were chirally orientated by glutamide groups, affording a unique orange-colored solution, with a visible absorption band at around 470 nm, having electronic circular dichroism (CD) signals (molar ellipticity [θ] = 0.58 × 105 deg cm2 dmol−1: absorption dissymmetry factors (g) = 5.2 × 10−3 at 512 nm). The G-V2+ could be reduced to its cation radical (G-V+˙) but retains its chiral assembly. After chemical reduction, the color change from orange to blueish violet, indicating an absorption band at approximately 560 nm, and the sign change of the CD signal from positive to negative ([θ] = −0.36 × 105 deg cm2 dmol−1; g = −2.9 × 10−3 at 580 nm) were observed in water. Subsequent oxidation re-introduces the G-V2+ chiroptical behavior before reduction.


 Please wait while we load your content...
Please wait while we load your content...