Size and surface-energy dependence of the adsorption/desorption equilibrium in ethanol electro-oxidation by Pd-nanoparticles. Theory and experiment†
Abstract
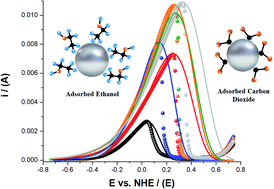
The relation between current and voltage in the electro-oxidation of ethanol by metal nanoparticles depends on experimental parameters like the applied potential, peak potential, temperature, the electron-transfer coefficient, and the number of molecules adsorbed at active sites on the nanoparticle surface. In this form, the oxidation current depends on the ability of the nanoparticles to adsorb the ethanol molecules. Though the Laviron model well describes this phenomenology, few studies focus on the dependence of the oxidation current on the size and surface properties of the metal nanoparticles. Here, we present an experimental and theoretical study that comprises the synthesis of palladium-based nanoparticles and the generalization of the Laviron model that allows determining the dependence of the oxidation current on the size, surface energy, and adsorption–desorption properties of the nanoparticles for the ethanol oxidation. The determination of the adsorption–desorption equilibrium and the electro-oxidation current dependence with the physicochemical properties of the materials was carried out by electrochemical characterization.


 Please wait while we load your content...
Please wait while we load your content...