Copper-assisted preparation of pyridinyl sulfonate esters from hydroxypyridines and sodium sulfinates†
Abstract
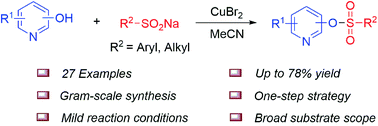
An efficient and powerful copper-assisted method for the effective conversion of a broad range of hydroxypyridines and sodium sulfinates into their corresponding pyridinyl tosylates was developed. Key features of this base- and ligand-free protocol include using the cheap and readily available CuBr2 as a medium and the use of sodium sulfinates as formal sulfonylation reagents. A variety of functional pyridinyl tosylates could be formed with good yields, which can easily be converted into C–C and C–N bond-containing compounds.


 Please wait while we load your content...
Please wait while we load your content...