Functionalized quinolizinium-based fluorescent reagents for modification of cysteine-containing peptides and proteins†
Abstract
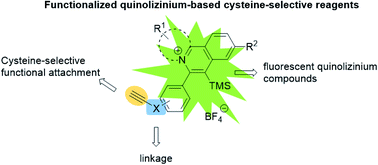
A series of quinolizinium-based fluorescent reagents were prepared by visible light-mediated gold-catalyzed cis-difunctionalization between quinolinium diazonium salts and electron-deficient alkyne-linked phenylethynyl trimethylsilanes. The electron-deficient alkynyl group of the quinolizinium-based fluorescent reagents underwent nucleophilic addition reaction with the sulfhydryl group on cysteine-containing peptides and proteins. The quinolizinium-based fluorescent reagents were found to function as highly selective reagents for the modification of cysteine-containing peptides and proteins with good to excellent conversions (up to 99%). Moreover, the modified BCArg mutants bearing cationic quinolizinium compounds 1b, 1d, 1e and 1h exhibit comparable activity in enzymatic and cytotoxicity assays to the unmodified one.


 Please wait while we load your content...
Please wait while we load your content...