Synthesis, solid state self-assembly driven by antiparallel π⋯π stacking and {⋯H–C–C–F}2 dimer synthons, and in vitro acetyl cholinesterase inhibition activity of phenoxy pendant isatins†
Abstract
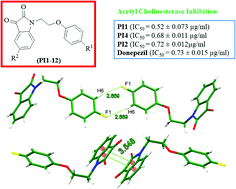
A series of novel phenoxy pendant isatins PI1–12 have been synthesized in excellent yields by a simple nucleophilic substitution reaction involving isatins and 1-(2-bromoethoxy)-4-substituted benzenes, and characterized by their FT-IR, 1H NMR, 13C NMR and GC-MS data, and in the case of PI4 by its single crystal X-ray analysis. The solid-state structure of PI4 showed an intriguing and unique 1D-supramolecular chain-based self-assembled structure, the driving force of which is mainly the strong antiparallel π⋯π stacking and {⋯H–C–C–F}2 dimer synthons. This compound not only highlights the potential of the isatin moiety in forming strong antiparallel π⋯π stacking interactions but also provides a platform to have considerable insight into the nature, strength and directionality of much debated π–π and C–H⋯F–C interactions. The in vitro biological studies revealed that three phenoxy pendant isatins PI1, PI2 and PI4 are highly potent inhibitors of acetylcholinesterase enzyme with IC50 values of 0.52 ± 0.073 μg ml−1, 0.72 ± 0.012 μg ml−1 and 0.68 ± 0.011 μg ml−1, respectively, showing comparable activity to the standard drug, donepezil (IC50 = 0.73 ± 0.015 μg ml−1). A simple and efficient synthesis of phenoxy pendant isatins PI1–12 from inexpensive and commercially available starting materials, and their high potential of acetyl cholinesterase inhibition provide an attractive opportunity to find more effective medication for Alzheimer's disease (AD).


 Please wait while we load your content...
Please wait while we load your content...