A thiadiazolopyridine-functionalized Zr(iv)-based metal–organic framework for enhanced photocatalytic synthesis of tetrahydroquinolines under visible light†
Abstract
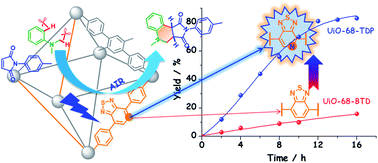
Metal–organic framework (MOF) materials provide a versatile and promising platform for constructing heterogeneous photocatalysts with applications in organic transformations. One of the methods for enhancing MOFs' performance in photocatalysis relies on the elaborate design and functionalization of organic linkers. Here we reported a photoactive thiadiazolopyridine (TDP) moiety functionalized UiO-68 isoreticular Zr(IV)-based MOF (denoted as UiO-68-TDP) that was synthesized by the de novo approach of mixed dicarboxylate struts. Under blue LED irradiation and in an open air atmosphere, MOF UiO-68-TDP exhibited a largely higher photocatalytic activity for the synthesis of tetrahydroquinolines by oxidative annulation reaction between N,N-dimethylanilines and maleimides, in comparison to the benzothiadiazole decorated analogue MOF. Besides, UiO-68-TDP can be reused at least three times without significant loss of its photocatalytic activity and its framework was well maintained after these cycles. Furthermore, the related mechanism involving reactive oxygen species was properly proposed.


 Please wait while we load your content...
Please wait while we load your content...