Ag2Mo2O7: an oxide solid-state Ag+ electrolyte†
Abstract
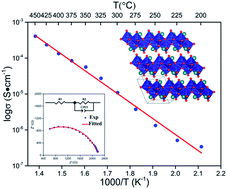
Ag2Mo2O7 powders and micro-crystals were prepared at 400 °C for 24 h and 500 °C for 6 h using solid-state reactions. The Ag2Mo2O7 samples crystalized in a triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group with the cell parameters a = 6.0972(1) Å, b = 7.5073(1) Å, c = 7.6779(2) Å, α = 110.43(1)°, β = 93.17(1)°, γ = 113.51(1)°, and V = 294.17(1) Å3 from Rietveld refinements. Ag2Mo2O7 powder is homogeneous with size of 2–8 μm and the ceramic pellets are in good sintering conditions with a relative density ∼93%. The indirect band gaps Eg(i) of Ag2Mo2O7 from reflectance measurements and DFT calculations are 2.63(1) and 1.80 eV. The vibrational modes of Ag2Mo2O7 were investigated by first-principles (DFT) calculations and Raman spectrum measurements with 24 of 33 predicted Raman modes recorded. According to DOS analyses, the valence bands (VB) of Ag2Mo2O7 are mainly constituted of O-2p and Ag-4d orbitals, while the conduction bands (CB) are mainly composed of Mo-4d and the O-2p orbitals. Regarding the impedance analysis, Ag2Mo2O7 is a silver oxide ion electrolyte with a conductivity of ∼5 × 10−4 S cm−1 at 450 °C. The carrier activation energy of Ag2Mo2O7 is 0.88(3) eV from the temperature dependent conductivity measurements.
space group with the cell parameters a = 6.0972(1) Å, b = 7.5073(1) Å, c = 7.6779(2) Å, α = 110.43(1)°, β = 93.17(1)°, γ = 113.51(1)°, and V = 294.17(1) Å3 from Rietveld refinements. Ag2Mo2O7 powder is homogeneous with size of 2–8 μm and the ceramic pellets are in good sintering conditions with a relative density ∼93%. The indirect band gaps Eg(i) of Ag2Mo2O7 from reflectance measurements and DFT calculations are 2.63(1) and 1.80 eV. The vibrational modes of Ag2Mo2O7 were investigated by first-principles (DFT) calculations and Raman spectrum measurements with 24 of 33 predicted Raman modes recorded. According to DOS analyses, the valence bands (VB) of Ag2Mo2O7 are mainly constituted of O-2p and Ag-4d orbitals, while the conduction bands (CB) are mainly composed of Mo-4d and the O-2p orbitals. Regarding the impedance analysis, Ag2Mo2O7 is a silver oxide ion electrolyte with a conductivity of ∼5 × 10−4 S cm−1 at 450 °C. The carrier activation energy of Ag2Mo2O7 is 0.88(3) eV from the temperature dependent conductivity measurements.


 Please wait while we load your content...
Please wait while we load your content...