Fundamental curiosity of multivicinal inter-halide stereocenters†
Abstract
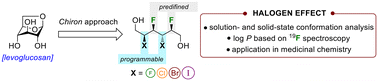
Although multivicinal inter-halide alkane units are represented in several natural products, many questions remain unanswered regarding the synthesis and physical properties of these unique molecules. Over the past decades, studies were directed towards the multivicinal fluoroalkane motifs, but other organohalogens have not been investigated. Herein, we present the first synthesis of complex 2,3,4-trihalohexanetriols and 2,3,4,5-tetrahalohexanediols using a Chiron approach. The dominant solution-state structure was established by J-based configurational analysis and the lipophilicity was assessed with a log P determination method based on 19F NMR spectroscopy. Two representative trihalogenated alkanes have been incorporated into piperidines of pitolisant, a histamine 3 receptor antagonist. This study unravels the striking impact of a single halogen on the physical properties, such as solution-state conformations, of inter-halide alkane motifs.


 Please wait while we load your content...
Please wait while we load your content...