Pd/Cu catalyzed carbonylation of α-aminoaryl-tethered alkylidenecyclopropanes: synthesis of furoquinoline derivatives†
Abstract
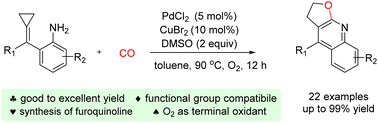
A Pd/Cu catalyzed carbonylation of α-aminoaryl-tethered ACPs for the synthesis of furoquinoline derivatives has been developed. Oxygen was used as the terminal oxidant in this reaction. A range of furoquinoline derivatives were efficiently prepared in good to excellent yields via the incorporation of a carbonyl group into the product with the cleavage of the proximal C–C bond of the ACPs.


 Please wait while we load your content...
Please wait while we load your content...