Palladium-catalyzed desulfonylative aminocarbonylation of benzylsulfonyl chlorides with o-aminobenzaldehydes/o-aminoacetophenones for the synthesis of quinoin-2(1H)-ones†
Abstract
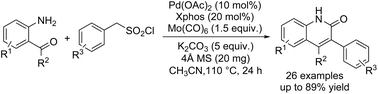
A straightforward and efficient synthesis of quinoin-2(1H)-ones has been explored via a palladium-catalyzed desulfonylative aminocarbonylation of benzylsulfonyl chlorides with o-aminobenzaldehydes/o-aminoacetophenones. With benzylsulfonyl chlorides as the C(sp3) electrophiles, a variety of quinoin-2(1H)-ones were obtained in moderate to high yields.


 Please wait while we load your content...
Please wait while we load your content...