Nucleophilic aromatic substitution approach to phosphanyl-substituted diboraanthracenes: biphilic compounds with tunable electron affinities†
Abstract
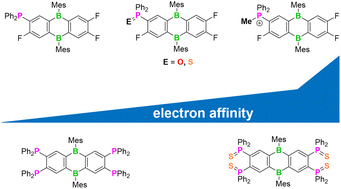
9,10-Dimesityl-9,10-dihydro-9,10-diboraanthracenes (Mes2DBAs) bearing one (1), two (2), or four (3) Ph2P substituents were prepared from Ph2PSiMe3 and tetrafluorinated Mes2DBAs via a nucleophilic aromatic substitution protocol. Using compound 1 as the model system, it was shown that P-oxidation (4), -sulfurization (5), or -methylation ([6]OTf) leads to a gradual anodic shift in the redox potentials of the compounds to an E1/2 value of ultimately −1.24 V (CH2Cl2; vs. FcH/FcH+). An exhaustive P-sulfurization is also possible for 2 and 3, and affords the Ph2P(S)-substituted species 7 and 8 with E1/2 values of −1.33 and −1.23 V, respectively. Fourfold P-methylation of 3 with MeOSO2CF3 (MeOTf) furnishes the salt [10](OTf)2, in which each of the two Mes substituents previously bonded to the B centers is replaced by two [OTf]− ligands. Late-stage P derivatization of Ph2P-carrying Mes2DBAs is thus a simple and effective means of tuning their redox properties and Lewis acidities.


 Please wait while we load your content...
Please wait while we load your content...