Pyrene and triphenylamine substituted cyanostyrene and cyanostilbene derivatives with dual-state emission for high-contrast mechanofluorochromism and cell imaging†
Abstract
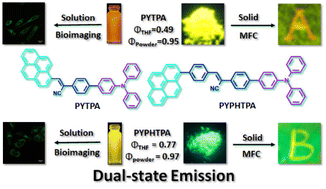
Dual-state emission (DSE) molecules overcome the limitations of trap-controlled quenching (TCQ) and solid-state luminescence enhancement (SLE), with the capacity to broaden the practical applications of luminescent materials. Herein, through introducing pyrene and TPA units into the α-cyanostyrene/cyanostilbene skeleton, two compounds (PYTPA and PYPHTPA) exhibiting DSE with a high quantum yield both in solution (ΦPYTPA = 0.49; ΦPYPHTPA = 0.77) and the solid state (ΦPYTPA = 0.95; ΦPYPHTPA = 0.97) have been synthesized. Both the compounds display high-contrast mechanofluorochromism. The spectral shifts (ΔEMCF) are 0.199 eV (ΔλPYTPA = 50 nm) and 0.248 eV (ΔλPYPHTPA = 55 nm) for PYTPA and PYPHTPA, respectively. Additionally, PYTPA and PYPHTPA were successfully applied for imaging in living HeLa cells. This work reveals that cyanostyrene/cyanostilbene-based DSEgens are promising materials for use in information storage and bioimaging.


 Please wait while we load your content...
Please wait while we load your content...