Gold-catalyzed formal (3 + 2) and (4 + 2) cycloaddition reactions using propiolates: assembly of 2,3-dihydrofurans and 3,4-dihydropyrans via a multistep cascade process†
Abstract
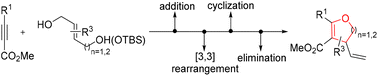
Herein, we report a gold-catalyzed formal dipolar cycloaddition approach toward dihydrofurans and dihydropyrans using polarized alkynes (propiolates) as dipolarophiles and butenediol or pentenediol derivatives as 1,3- or 1,4-dipoles. The reaction proceeded via a multistep cascade process with a broad substrate scope. A temporary silyl protection strategy was used to solve the regioselectivity problem of unsymmetrical diols. Moreover, the reaction could be readily scaled up and the products could be further elaborated into a diverse collection of highly substituted and functionalized cyclic complex structures.


 Please wait while we load your content...
Please wait while we load your content...