Exploring the regioselectivity of the cyanoalkylation of 3-aza-1,5-dienes: photoinduced synthesis of 3-cyanoalkyl-4-pyrrolin-2-ones†
Abstract
A visible-light-induced regioselective cyanoalkylalkenylation of 1,5-dienes with oxime esters as the alkyl radical precursors is reported. Such a radical cascade proceeds with high yields and a broad substrate scope under mild conditions to provide 3-cyanoalkyl-4-pyrrolin-2-ones in one step from readily available N-vinylacrylamides. It is readily scalable to the gram scale, and could be induced by natural sunlight as a sustainable light source.
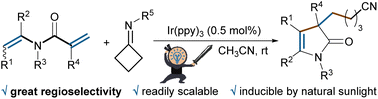


 Please wait while we load your content...
Please wait while we load your content...