Switchable synthesis of 1,4-bridged dihydroisoquinoline-3-ones and isoquinoline-1,3,4-triones through radical oxidation of isoquinolinium salts with phenyliodine(iii) diacetate†
Abstract
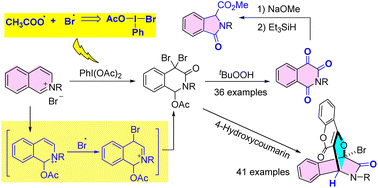
A metal-free switchable synthesis of 1,4-bridged dihydroisoquinoline-3-ones and isoquinoline-1,3,4-triones through the oxidation of isoquinolinium salts is described. 1,4-Bridged dihydroisoquinolin-3-ones were constructed for the first time via the sequential oxidation/annulation of isoquinolinium salts with 4-hydroxycoumarins. Meanwhile, the iodine(III)-oxidized intermediates of isoquinolinium salt could be converted to isoquinoline-1,3,4-triones in high yields by treatment with tert-butyl hydroperoxide. These site-selective transformations rely on an iodine(III)-mediated dual radical addition/radical coupling strategy and prove that the combination of the bromide anion and phenyliodine(III) diacetate is a useful carrier of relatively stable bromine radicals and highly labile carboxyl radicals.


 Please wait while we load your content...
Please wait while we load your content...