Bio-inspired construction of a tetracyclic ring system with an avarane skeleton: total synthesis of dactyloquinone A†
Abstract
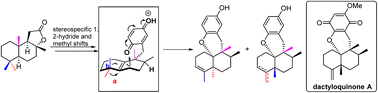
The asymmetric synthesis of the marine meroterpenoid dactyloquinone A was accomplished for the first time. The key advanced intermediate, a 6/6/6/6 tetracyclic core derivative, was constructed by a bio-inspired Lewis acid-catalyzed cyclization reaction, which drove the migration of the methyl groups of two different configurations at the C-4 site by 1,2-rearrangement from the aureane skeleton to the avarane skeleton. This strategy sets the stage for the synthetic exploration of other members of this family of natural products.


 Please wait while we load your content...
Please wait while we load your content...