Traceless proton aided regioselective C(sp2)–C(sp2) construction to synthesize C6-acylated purines and purine nucleosides without metal catalysts†
Abstract
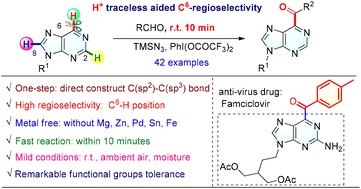
Although three C–H bonds (C2–H, C6–H, and C8–H) and four nitrogen atoms (N1, N3, N7, and N9) exist in the structure of purines, the traceless H+ aided highly regioselective radical reaction for the synthesis of C6-acylated purines and purine nucleosides has been developed at room temperature within 10 min. This reaction avoids metal catalysts and harsh reaction conditions and features excellent functional group compatibility, broad substrate scope, and good C6-regioselectivity. H+ is green, environmentally friendly, and traceless and does not require additional functional group modification before the reaction. Moreover, this procedure could be used to produce other functional purines, which are potentially of great importance in medicinal chemistry.


 Please wait while we load your content...
Please wait while we load your content...