Metal-free dearomatization reactions of naphthol-ynamides for the divergent and enantioselective synthesis of azaspirocycles†
Abstract
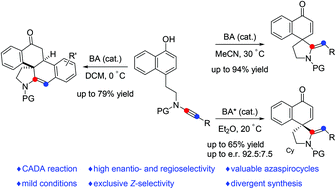
Dearomatization reactions of phenols and their derivatives have evolved as efficient and practical methods for the synthesis of functionalized spirocyclic enones in the past decades. Here, an efficient metal-free intramolecular dearomatization cyclization of readily available naphthol-ynamides has been developed, enabling practical, atom-economical and divergent access to two azaspirocyclic compounds in mostly good to excellent yields with a wide substrate scope. Moreover, such a catalytic asymmetric dearomatization reaction has also been achieved by employing a chiral Brønsted acid as a catalyst.
- This article is part of the themed collection: 2022 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...