Synthesis of dihydropyrazoles enabled by Pd-catalyzed carboamination of alkenyl hydrazones with alkenyl and aryl halides†
Abstract
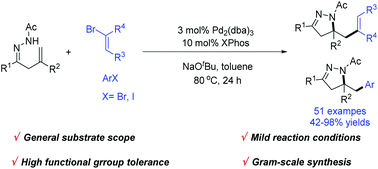
Herein, a novel and highly efficient strategy for the synthesis of dihydropyrazoles via the Pd-catalyzed carboamination reaction of β,γ-unsaturated hydrazones with alkenyl and aryl halides is demonstrated. The present methodology provides a practical protocol for accessing various substituted dihydropyrazoles with good yields and good functional group tolerance.


 Please wait while we load your content...
Please wait while we load your content...