Trimacrocyclic hexasubstituted benzenes for recognition of guanidinium and their anti-cancer and antimicrobial activities†
Abstract
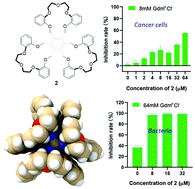
Notwithstanding the considerable advances in the field of biomaterials, there is still an ongoing quest for the development of new materials and strategies for anti-cancer and antimicrobial drug discovery. Herein, we detail the synthesis of two trimacrocyclic hexasubstituted benzenes (THBs, 1 and 2) via a Co2(CO)8-catalyzed [2 + 2 + 2] tricyclization synthetic strategy. Compounds 1 and 2 show a great propensity for complexing guanidinium cations, as inferred from 1H NMR spectroscopic studies and single-crystal X-ray crystallography, as well as DFT calculations and molecular dynamics simulations. Under solid–liquid extraction conditions, 1 and 2 were able to extract guanidinium cations from the solid state into the chloroform phase. The guanidinium recognition-induced promotion of anti-cancer and antimicrobial activities was further exemplified by the MTT cell viability assays and antimicrobial studies using receptor 2 in the presence of guanidinium chloride. This study provides insight into anti-cancer and antimicrobial drug discovery in a supramolecular way.


 Please wait while we load your content...
Please wait while we load your content...