Ru(iii)-catalyzed C4–H bond cyanoalkoxylation of 1-naphthylamine derivatives with azobisisobutyronitrile†
Abstract
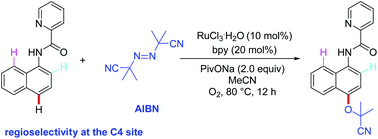
A simple and efficient protocol for ruthenium-catalyzed C4–H bond cyanoalkoxylation of 1-naphthylamine derivatives with AIBN was developed under an oxygen atmosphere. This reaction provides an efficient method for preparing cyano group-containing compounds in moderate to good yields, and exhibits broad functional group tolerance and regioselectivity. The obtained cyano group-containing products have significant potential value in organic synthesis, such as the easy removal of the directing group to form the corresponding naphthylamine derivatives and the conversion of the cyano group into an amide moiety by hydrolysis. Further mechanistic exploration indicated that this ruthenium-catalyzed version of cyanoalkoxylation is a radical process.


 Please wait while we load your content...
Please wait while we load your content...