Borane-catalyzed dehydrogenative C–C bond formation of indoles with N-tosylhydrazones: an experimental and computational study†
Abstract
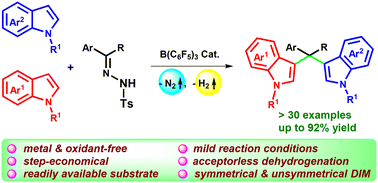
A novel dehydrogenative C–C bond formation of indoles and N-tosylhydrazones to give di(indolyl)methanes (DIMs) has been demonstrated using tris(pentafluorophenyl)borane as a catalyst. A wide range of functional groups can be tolerated under this metal-free protocol to afford symmetrical and most importantly unsymmetrical DIMs. Extensive experimental investigations like the kinetic profile, Hammett plot, determination of reaction order, and HRMS analysis for intermediates along with computational study suggest that the reaction proceeds through sequential intermolecular C–C/C–C bond formation with indoles and borane adducts of N-tosylhydrazones followed by acceptorless dehydrogenation for the access of bioactive DIMs.


 Please wait while we load your content...
Please wait while we load your content...