Alkynyl-induced construction of stereodefined polysubstituted conjugated enynes via Pd-catalyzed allylic arylations†
Abstract
The alkynyl-induced stereoselective construction of polysubstituted enynes via Pd-catalyzed allylic arylations is described. The interaction between the alkynyl group and the Pd-allyl fragment is proposed to be the key toward the desired products with exclusive (Z)-configurations. This methodology enables the construction of a number of structurally diverse and otherwise synthetically challenging (Z)-polysubstituted enynes under user-friendly conditions.
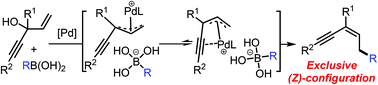


 Please wait while we load your content...
Please wait while we load your content...