Regio- and stereoselective electrochemical selenoalkylation of alkynes with 1,3-dicarbonyl compounds and diselenides†
Abstract
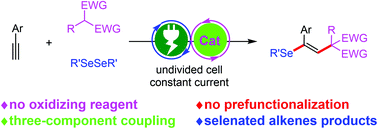
A regio- and stereoselective electrochemical approach for the seleno(monofluoro)alkylation of alkynes with 1,3-dicarbonyl compounds and diselenides has been developed. The electrosynthesis method utilizes C–H substrates as radical precursors and the source of carbon atoms, avoiding dependence on pre-functionalized substrates. A series of selenated alkenes are prepared with satisfactory outcomes under noble metal-free and oxidizing reagent-free conditions.


 Please wait while we load your content...
Please wait while we load your content...