Assembly of tetracyclic tetrahydrocarbazoles via a visible-light promoted cascade process†
Abstract
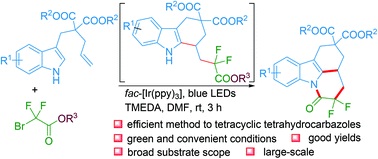
We disclose an efficient method for the synthesis of tetracyclic tetrahydrocarbazoles via a visible-light promoted cascade reaction of alkene tethered indoles and bromodifluoroacetate esters. This reaction involves the formation of two C–C bonds and one C–N bond and proceeds via a key reactive tetrahydrocarbazole intermediate and dual cyclization. The reaction features mild conditions, wide substrate scope, and good chemical yields, and provides a new strategy for the synthesis of tetracyclic tetrahydrocarbazole derivatives. The synthetic utility of this reaction was further demonstrated by the large-scale synthesis and reduction of the obtained tetracyclic tetrahydrocarbazole product.


 Please wait while we load your content...
Please wait while we load your content...