Visible-light-driven photoredox-catalyzed C(sp3)–C(sp3) cross-coupling of N-arylamines with cycloketone oxime esters†
Abstract
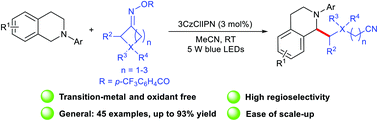
A novel photoredox-catalyzed C(sp3)–C(sp3) cross-coupling between N-arylamines and cycloketone oxime esters under mild conditions has been accomplished. The redox-neutral reaction proceeds with good functional group tolerance and excellent regioselectivity without any extra base or ligand control. In addition, this approach was successfully applied for the late-stage modification of short peptides and analogues.
- This article is part of the themed collection: FOCUS: Radical-involved chemical transformations


 Please wait while we load your content...
Please wait while we load your content...