Nickel-catalyzed multicomponent reaction of dinitriles and hydrazine hydrochlorides with boronic acids: access to 1,3-diaryl-1H-pyrazol-5-amines and 4,5-dihydropyridazin-3(2H)-ones†
Abstract
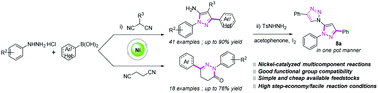
Developing efficient methods to accommodate azaheterocycles has been a hotspot field in organic synthesis. Herein, we disclose a facile and expeditious avenue to synthesize 1,3-diaryl-1H-pyrazol-5-amines and 4,5-dihydropyridazin-3(2H)-ones via a nickel-catalyzed addition, condensation, and annulation sequence of commercially available dinitriles and boronic acids with hydrazine hydrochlorides. The conversion features a high step economy and good functional group tolerance. The synthetic utility of this methodology is demonstrated by scaled-up reaction and construction of a biologically active triazole biheteroaryl skeleton in a one-pot manner from simple feedstocks.


 Please wait while we load your content...
Please wait while we load your content...