Formal dual C(sp2)–H cross-dehydrogenative C–O bond formation to construct highly functionalized diaryl ethers with O2†
Abstract
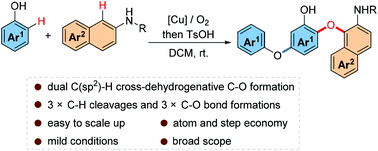
A formal dual C(sp2)–H cross-dehydrogenative C–O bond formation between phenols and N-substituted naphthylamines to expediently assemble diversely functionalized diaryl ethers bearing hydroxyl and amino functionalities with excellent atom and step economy was developed under mild conditions. The reaction features three C–H cleavages and three C–O bond formations in one pot. In addition a rare C(sp2)–O bond formation rather than the conventional C–C bond formation took place by addition to a carbonyl O atom of the in situ generated ortho-quinone.


 Please wait while we load your content...
Please wait while we load your content...