Chemical and chemoenzymatic stereoselective synthesis of β-nucleosides and their analogues
Abstract
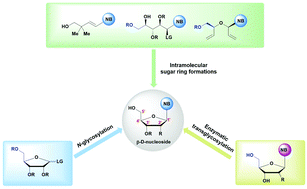
β-Nucleosides are fundamental building blocks of biological systems and are widely used as therapeutic agents for the treatment of cancer and viral infections, among others. In the last two years, nucleoside analogues have been investigated with renewed urgency in the search for agents that are effective against SARS-CoV-2, the cause of the ongoing global pandemic of COVID-19. This has resulted in an explosion of activities in the field of β-nucleoside synthesis. This review summarizes the historical perspective and the recent advances in the stereoselective synthesis of β-nucleosides and their analogues. The synthetic strategies to obtain β-nucleosides can be divided into three categories: (1) N-glycosylation; (2) intramolecular sugar ring formation; and (3) enzymatic transglycosylation.
- This article is part of the themed collection: 2022 Organic Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...