Construction of diverse C–S/C–Se bonds via nickel catalyzed reductive coupling employing thiosulfonates and a selenosulfonate under mild conditions†
Abstract
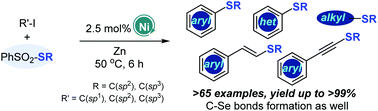
A nickel-catalyzed reductive cross coupling between organic iodides and thiosulfonates and a selenosulfonate under mild conditions is disclosed. This practical method provides a facile access to a series of unsymmetrical thioethers with low catalyst loading, good functional group tolerance, and excellent chemo-selectivity. Notably, the synthetic applications of the approach feature scaling-up of reactions, late-stage modification of pharmaceuticals, and preparation of various useful targeted compounds, including sulfoximine, bipyridine, and vortioxetine. Primary mechanistic studies showed that a radical pathway was involved. Moreover, diverse C–S/C–Se bond formations were achieved under mild reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...