Palladium-catalyzed direct γ-C(sp3)–H arylation of β-alkoxy cyclohexenones: reaction scope and mechanistic insights†
Abstract
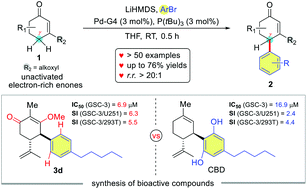
The direct γ-C(sp3)-arylation of unactivated electron-rich enones has been a long-standing challenge. Herein, a mild Pd-catalyzed method for direct γ-C(sp3)-arylation of various unactivated β-alkoxy cyclohexenones is reported. The method is not only tolerated well with many ortho-hetero substituted aryl bromides but also with the sterically hindered ortho-dimethoxy or rigid ortho-diaryl aryl bromides. Mechanistic studies suggest the tunneling is likely to govern the [1,5]-H shift of the α′-dienolate-PdArL intermediate and consequently forms the γ-arylated product, therefore exhibiting an excellent γ-site selectivity. Finally, our method enables the facile synthesis of CBD-like compounds with selective anti-proliferative effects against glioma stem cells.


 Please wait while we load your content...
Please wait while we load your content...