Visible-light mediated cross-coupling of aryl halides with sodium sulfinates via carbonyl-photoredox/nickel dual catalysis†
Abstract
Photoinduced nickel-catalyzed cross-coupling of arylsulfinates (ArSO2−) with (hetero)aryl halides (Ar′–X) via visible light photoexcitation of 2-chloro-thioxanthen-9-one (Cl-TXO) has been achieved in moderate to excellent yields. This photocoupling exhibited a broad substrate scope with good functional group tolerance to give diarylsulfones. The mechanism involves oxidative addition of Ar′–X to Ni(0), oxidation with 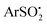 derived from single-electron-oxidation of ArSO2− by photoexcited Cl-TXO*, reductive elimination and release of the product diaryl sulfone and NiI catalyst. The photoredox and nickel catalytic cycles interact through electron transfer from the single-electron-reduced Cl-TXO ([Cl-TXO]˙−) to the NiI species.
derived from single-electron-oxidation of ArSO2− by photoexcited Cl-TXO*, reductive elimination and release of the product diaryl sulfone and NiI catalyst. The photoredox and nickel catalytic cycles interact through electron transfer from the single-electron-reduced Cl-TXO ([Cl-TXO]˙−) to the NiI species.



 Please wait while we load your content...
Please wait while we load your content...