Chemo-selective control of Ritter-type reaction by coordinatively unsaturated inorganic salt hydrates†
Abstract
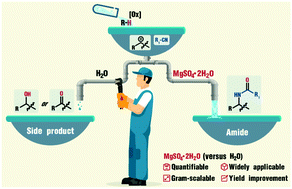
The Ritter-type reaction, without relying on a pre-installed functional group, is a highly efficient tool for the construction of amides. However, the intrinsic chemo-selectivity that determines the generation of amides or byproducts limits the efficiency and yield of the reaction. From 68 different types of hydrates, we found that a coordinatively unsaturated inorganic salt hydrate, MgSO4·2H2O, controlled chemo-selectivity and eliminated the shortcomings of other synthesis approaches. To rationalize the differences in selectivity of inorganic salt hydrates, we analyzed their corresponding water content, alkalinity, anions, and cations. MgSO4·2H2O was used with diverse scaffolds and C–H oxygenations, which demonstrated its generality in synthetic utility. Because it is readily available and it significantly improves yield, we expect that MgSO4·2H2O will have broad application for Ritter-type reactions.


 Please wait while we load your content...
Please wait while we load your content...