Catalytic properties of 4,5-bridged proline methano- and ethanologues in the Hajos–Parrish intramolecular aldol reaction†
Abstract
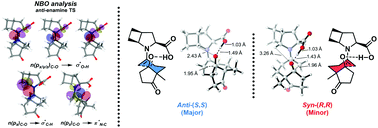
The catalysis of the Hajos–Parrish reaction by cis- and trans-4,5-ethano-proline was explored experimentally and computationally with DFT (ωB97X-D and MN15) and DLPNO-CCSD(T). Both of the new catalysts are as active as proline or cis- and trans-4,5-methano-proline, with the trans-ethano-proline being as enantioselective as proline. A thorough theoretical analysis of the electronic factors influencing catalysis is reported.


 Please wait while we load your content...
Please wait while we load your content...