Tandem cyclization/arylation of diaryliodoniums via in situ constructed benzoxazole as a directing group for atom-economical transformation†
Abstract
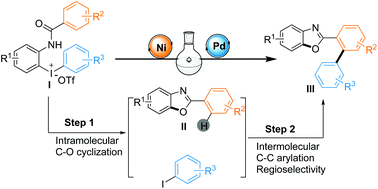
Linear diaryliodoniums often undergo only single arylation and leave equivalent aryl iodide as waste. Herein, we demonstrate that linear unsymmetrical diaryliodoniums could be tuned by a dual nickel/palladium metal system to accomplish one-pot cyclization/arylation. Of note, the in situ generated benzoxazole motifs could then be utilized as directing groups for subsequent C–H activated arylation from the often unwanted aryl iodide, exhibiting good reactions with atom economy and regioselectivity.


 Please wait while we load your content...
Please wait while we load your content...