Electrochemically-mediated C–H functionalization of allenes and 1,3-dicarbonyl compounds to construct tetrasubstituted furans†
Abstract
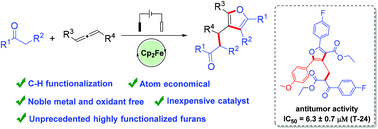
C–H functionalization of allenes is an available strategy to construct heterocyclic compounds. Herein we propose a sus-tainable electrocatalytic C–H activation for the synthesis of novel highly functionalized tetrasubstituted furans, which is accomplished by intermolecular cyclization of allenes and 1,3-dicarbonyl compounds. This electrochemical allene annulation avoids the requirement for stoichiometric oxidants and noble metal catalysts by using Cp2Fe as redox catalyst. The cytotoxicity of synthesized tetrasubstituted furans was investigated by MTT assay, and one of the compounds showed good antitumor activity in vitro.


 Please wait while we load your content...
Please wait while we load your content...