Effect of potential difference between the central and terminal metals on the electron communication in an Fe–Ru–Fe cyanidometal-bridged mixed valence system†
Abstract
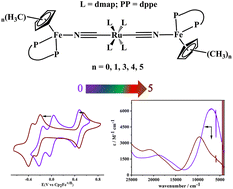
A series of trimetallic cyanidometal-bridged compounds with the formula [CpMexFe–NC–Ru–CN–FeCpMex]n+, Nn+ (n = 2, 3 and 4; N = 1, x = 0; N = 2, x = 1; N = 3, x = 3; N = 4, x = 4; N = 5, x = 5), were synthesized and fully characterized. The effect of the potential difference between the central and terminal metals on the electron communication in these trinuclear mixed valence systems was investigated. N3+ and N4+ are the one- and two-electron oxidation products of N2+. Electrochemical measurements show the presence of electronic interactions between the two terminal Fe atoms. The electronic properties of N3+ and N4+ were studied and compared using experimental spectroscopy and time-dependent density-functional calculations. As the number of methyl substituents on the cyclopentadiene ligand increases, the potential difference between the central and terminal redox centers increases and thus, the electron communication between the terminal Fe redox centers or between the neighboring Fe and Ru redox centers becomes weaker. All the mixed valence compounds show localized character.


 Please wait while we load your content...
Please wait while we load your content...