Promoting oxygen reduction via crafting bridge-bonded oxygen ligands on a single-atom iron catalyst†
Abstract
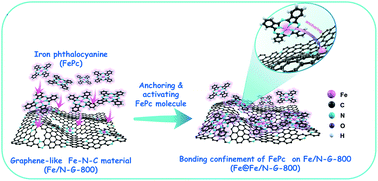
Single-atom Fe–N–C materials with Fe–N4 coordination structures, hailed as promising catalysts, are prohibited by the severe aggregation and migration of metal atoms. Although bonding-confinement strategies can be used to effectively regulate and strengthen the coordination of isolated metal atoms, the precise control of the coordination environment of metal centers remains a challenge. Herein, we report a rational strategy by which to bond iron phthalocyanine (FePc) on pre-synthesized Fe–N–C materials to further obtain anatomically dispersed Fe–N4 catalysts. The axial coordination of O-FeN4 sites to form a Fe–O–Fe bridge bond lowers the overpotential for the oxygen reduction reaction (ORR). Incorporation of the O atom stimulates the adsorbed O2 to obtain more electrons, thereby enhancing the adsorption and activation of O2. The catalyst demonstrates a half-wave potential of 0.866 V (versus RHE) and kinetic current density of 11.49 mA cm−2, significantly outperforming commercial Pt/C. The primary Zn–air battery assembled with such a catalyst exhibits a high current density of 136 mA cm−2 @ 1.0 V and a maximum power density of 205 mW cm−2, supporting its potential feasibility in practical applications. Our findings provide a new avenue for tuning the coordination environment of single-atom catalysts to enhance their ORR activity.


 Please wait while we load your content...
Please wait while we load your content...