Assembling lanthanide–transition metal clusters on TiO2 for photocatalytic nitrogen fixation†
Abstract
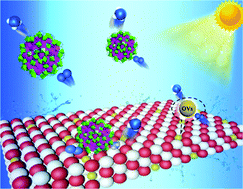
Ammonia synthesis using light with low energy consumption offers an effective solution for energy saving and environmental protection. Herein, an abundant oxygen vacancy photocatalyst was synthesized via the integration of lanthanide–transition metal (4f–3d) clusters Ln52Ni56 on the TiO2 surface. The investigation of photocatalytic nitrogen fixation showed that Ln52Ni56 not only acts as a tool to improve charge separation but also enriches oxygen vacancies. Multiple synergies resulted in a photocatalytic nitrogen fixation efficiency of up to 800 μmol h−1 g−1 with the direct utilization of nitrogen and water without any sacrificial agents or co-catalysts. Electron paramagnetic resonance spectroscopy was conducted to investigate the mechanism of oxygen vacancy inactivation and recovery. This study provides a reference for the construction of a photochemical nitrogen fixation catalyst driven by defect engineering.
- This article is part of the themed collections: FOCUS: Metal and Metal-Containing Clusters and FOCUS: Photocatalysis


 Please wait while we load your content...
Please wait while we load your content...