Excessive consumption mechanism of hydrazine in the reaction with ReO4−: Re species evolution and ReO2·nH2O-catalyzed decomposition†
Abstract
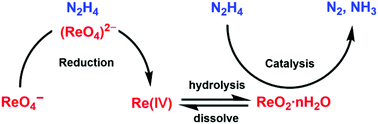
The reaction between pertechnetate (99TcO4−) and hydrazine is considered to be that of a complex inorganic chain in the PUREX process, in which hydrazine is excessively consumed with a high stoichiometric ratio of [N2H4]/[Tc]. Due to the ambiguity of the low-valent Tc species in the catalytic process, the consumption mechanism remains to be further optimized. In this study, perrhenate (ReO4−) was used to simulate the redox behavior of TcO4− with hydrazine, and the consumption mechanism of hydrazine was examined. In batch experiments where different initial concentrations of ReO4− and hydrazine were used, the kinetic profile of hydrazine consisted of an induction period, a rapid reaction stage, and a termination stage. There was only a slight decrease in the hydrazine concentration during the induction period, and the length of the induction period was strongly dependent on the initial concentrations of hydrazine and ReO4−. Then, the hydrazine concentration significantly decreased during the rapid reaction stage, and was completely consumed after the termination stage. Because excess hydrazine was over-consumed, the Re species in the reaction were investigated to study the hydrazine consumption mechanism. ReO4− was slowly reduced to ReO42− and Re(IV) species during an induction period, with an absorption peak of 269 nm and 301 nm, respectively, and X-ray absorption fine structures (XAFs) also supported evidence of ReO42− and soluble Re(IV) species. Most Re(IV) species were hydrolyzed to ReO2·nH2O during the rapid reaction stage. Further experiments suggest that ReO2/ReO2·nH2O would catalyze the decomposition of hydrazine at room temperature, and lead to the excessive consumption of hydrazine in the reaction. Gas chromatography confirmed that hydrazine mainly decomposed into N2 and NH3.


 Please wait while we load your content...
Please wait while we load your content...