Enantioselective chiral sorption of 1-phenylethanol by homochiral 1D coordination polymers†
Abstract
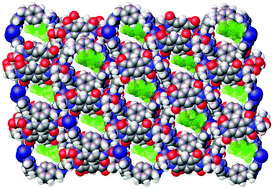
Enantioselective resolution by coordination polymers has the potential to provide a high degree of discrimination by virtue of well-defined pores and the possibility of identifying and modifying binding sites. Differences in enantioselective behaviour of two closely-related chiral 1D coordination polymers are shown to relate to their slight differences in structure. The coordination polymers, 1 and 2, contain an enantiopure dicarboxylate diimide ligand (AlaPmDI2−) alongside an achiral co-ligand, 1,4-bis(imidazol-1-yl-methyl)benzene (bix), with the substantiative difference between the two being replacement of one AlaPmDI2− by two chloride ligands. Despite being one-dimensional, both present small channels of intrinsic chirality. Compound 1 was found to exhibit modest enantioselectivity for (R)-1-phenylethanol (1-PE) from static sorption, while 2 did not show any enantioselectivity in its uptake, although still sorbed the guest. Single crystal data of 1-PE⊂1 indicates that the enantioselective preference is due to an array of interactions with the hydroxyl group of the (R)-enantiomer. Use of 1 in a pseudo-LC micro-column is able to provide enantio-enriched samples from a dynamic separation.


 Please wait while we load your content...
Please wait while we load your content...