Cyclopolymerizable and cyclopolymeric photoinitiators from diallyl amine and α-hydroxy ketones†
Abstract
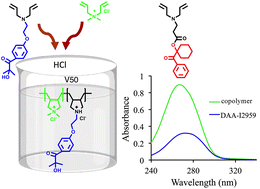
Two novel cyclopolymerizable photoinitiators (DAA-I2959 and DAA-I184) are synthesized by attachment of diallyl amine (DAA) to 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (Irgacure 2959 or I2959) and 1-hydroxycyclohexyl phenyl ketone (Irgacure 184 or I184); for free radical polymerization of (meth)acrylates. The copolymerization of DAA-I2959 with diallyldimethylammonium chloride (DADMAC) in the presence of hydrochloric acid gives a novel water soluble linear polymeric photoinitiator (PPI), p-DADMAC-co-DAA-I2959-QS (Mn = 40 000–60 000 D) with five-membered pyrrolidine rings in the main chain (first PI with a cyclic backbone in the literature). DAA-I2959, its copolymer and DAA-I184 have strong absorbances at 272, 266 and 244 nm (ε ∼ 14 705, effectively 8000 and 11 625), respectively, in methanol. These PIs can successfully initiate the photopolymerization of poly(ethylene glycol) diacrylate (PEGDA, Mn = 575 D) or 2-hydroxyethyl methacrylate (HEMA); DAA-I2959 exhibits a photoinitiating ability similar to I2959 and can also be used as a hydrogen donor for a Type II photoinitiating system. DAA-I184 shows much slower kinetics compared to I184. DAA-I2959 and p-DADMAC-co-DAA-I2959-QS exhibit fairly good migration stability (∼3.5 and 6.8 times higher) compared to I2959.


 Please wait while we load your content...
Please wait while we load your content...