Radical-disulfide exchange in thiol–ene–disulfidation polymerizations†
Abstract
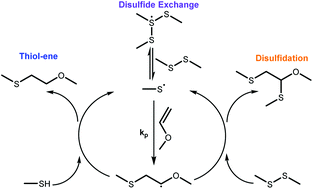
Radical-disulfide exchange reactions in thiol–ene–disulfide networks were evaluated for several structurally distinct thiol and disulfide containing monomers. A new dimercaptopropionate disulfide monomer was introduced to assess how different disulfide moieties affect the exchange process and how the dynamic exchange impacts polymerization. The stress relaxation rate for the disulfides studied herein was highly tunable over a narrow range of network compositions, ranging from 50% relaxation over 10 minutes to complete relaxation over a few seconds, by changing the thiol–disulfide stoichiometry or the disulfide type in the monomer. The thiol/disulfide monomer pair was shown to have significant influence on how radical-disulfide exchange impacts the polymerization rate, where pairing a more stable radical forming thiol (e.g. an alkyl thiol) with a less stable radical-forming disulfide (e.g. a dithioglycolate disulfide) reduces the rate of the thiol–ene reaction by over an order of magnitude compared to the case where those two radicals are of the same type. The variations in rates of radical-disulfide exchange with dithioglycolate and dimercaptopropionate disulfides had a significant impact on stress relaxation and polymerization stress, where the stress due to polymerization for the final dimercaptopropionate network was about 20% of the stress in the equivalent dithiogylcolate network under the same conditions. These studies provide a fundamental understanding of this polymerization scheme and enable its implementation in materials design.


 Please wait while we load your content...
Please wait while we load your content...