Radical homopolymerization of vinyl ethers activated by Li+-π complexation in the presence of CH3OLi and LiI†
Abstract
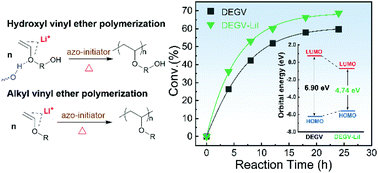
In this study, we develop a direct, thermally initiated radical homopolymerization of vinyl ethers mediated by lithium salts CH3OLi and LiI. In the case of vinyl ether monomers having a hydroxyl group, for example, diethyleneglycol monovinyl ether (DEGV), a high molecular weight poly(DEGV) (Mn = 18 700) is produced in a high yield (≥68%) with negligible acetal formation. Most importantly, this method further enables the radical polymerization of alkyl vinyl ether (IBVE) which has no hydroxyl group to form hydrogen bonds to facilitate the radical polymerization. There are no evident structural defects caused by side reactions such as β-scission and hydrogen abstraction as the poly(IBVE) yield is up to about 50% and Mn = 8500. Under similar conditions, the poly(IBVE) yield of the control run is only 16.2%. Density functional theory calculations suggest that the interaction between Li+ and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O of IBVE significantly reduces the HOMO–LUMO energy gap from 6.83 eV to 4.61 eV. In addition, the changes in 1H-NMR chemical shifts of CH2
C–O of IBVE significantly reduces the HOMO–LUMO energy gap from 6.83 eV to 4.61 eV. In addition, the changes in 1H-NMR chemical shifts of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–O– further support the existence of interaction between Li+ and C
CH–O– further support the existence of interaction between Li+ and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O. This cation-π complexation between Li+ and vinyl ether can effectively reduce the electron density of the vinyl group and as a result increases the stability of σ-radicals and suppresses unfavorable side reactions.
C–O. This cation-π complexation between Li+ and vinyl ether can effectively reduce the electron density of the vinyl group and as a result increases the stability of σ-radicals and suppresses unfavorable side reactions.


 Please wait while we load your content...
Please wait while we load your content...