Iron-containing poly(ionic liquid) membranes: a heterogeneous Fenton reaction and enhanced anti-fouling ability†
Abstract
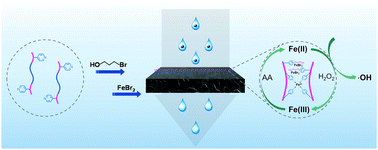
Poly(ionic liquid)s (PILs) are widely used to improve the anti-fouling ability of membranes due to their high charge density and excellent hydrophilicity. However, it is difficult to eradicate the irreversible pollutants deposited inside the pores of membranes. In this study, an iron-containing PIL (Fe-PIL) membrane was constructed for the first time to enhance the anti-fouling property of a membrane via a heterogeneous Fenton reaction. Cu(0)-mediated reversible deactivation radical polymerization was used to synthesize polysulfone-based block copolymers with pyridine pendant groups, which were then quaternized for coordination with Fe(II) bromide to yield the targeted Fe-PILs. The presence of Fe-PILs enabled the heterogeneous Fenton reaction within the membrane pores and exhibited a wide range of pH tolerance, which could altogether remove contaminants within minutes. The membrane showed good anti-fouling performance and reusability. In addition, membrane filtration and the heterogeneous Fenton reaction exhibited a synergistic effect due to the confinement effect, improving the permeability and rejection of the membrane. This research developed a novel synthetic strategy and catalytic function of Fe-PILs for advanced membranes.


 Please wait while we load your content...
Please wait while we load your content...