Structure revision and chemical synthesis of ligandrol's main bishydroxylated long-term metabolic marker†
Abstract
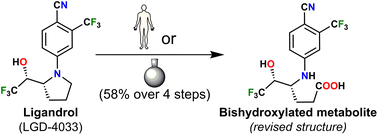
Although the main bishydroxylated long-term metabolite of the WADA-banned anabolic agent ligandrol (LGD-4033) is an important metabolic marker, it is not readily available in sufficient quantities to facilitate the development and validation of related analytical protocols or sensors. A chemically more robust structure was postulated as an alternative to the one previously established. The NMR spectra of the synthesized material and its LC–HRMS comparison with a relevant metabolic sample support the proposed structural revision.


 Please wait while we load your content...
Please wait while we load your content...