Substitution pattern in ruthenium octa-n-butoxyphthalocyanine complexes influence their reactivity in N–H carbene insertions†
Abstract
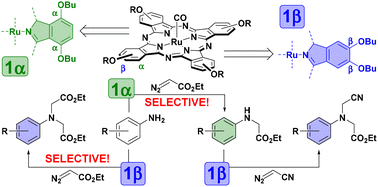
Ruthenium phthalocyanine complexes bearing n-OBu substituents in the peripheral or non-peripheral positions are efficient catalysts for the selective double or single carbene insertion to the amine N–H bonds. This complementary reactivity of two Ru complexes can be used for the synthesis of asymmetric tertiary amines and diamines bearing different substituents and has been demonstrated by two examples of readily available primary amines using different carbene precursors in successive reactions.


 Please wait while we load your content...
Please wait while we load your content...